Advertisement
Help Keep Boards Alive. Support us by going ad free today. See here: https://subscriptions.boards.ie/.
If we do not hit our goal we will be forced to close the site.
Current status: https://keepboardsalive.com/
Annual subs are best for most impact. If you are still undecided on going Ad Free - you can also donate using the Paypal Donate option. All contribution helps. Thank you.
If we do not hit our goal we will be forced to close the site.
Current status: https://keepboardsalive.com/
Annual subs are best for most impact. If you are still undecided on going Ad Free - you can also donate using the Paypal Donate option. All contribution helps. Thank you.
https://www.boards.ie/group/1878-subscribers-forum
Private Group for paid up members of Boards.ie. Join the club.
Private Group for paid up members of Boards.ie. Join the club.
Hi all, please see this major site announcement: https://www.boards.ie/discussion/2058427594/boards-ie-2026
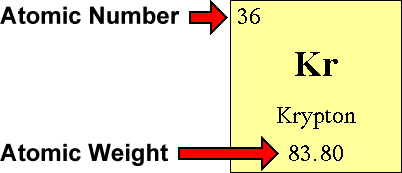
Let's count electrons.
-
30-05-2008 05:39PM#1
Comments
-
-
-
-
-
-
Advertisement
-
-
-
-
-
-
Advertisement
-
-
-
-
-
-
-
-
-
-
-
Advertisement
-
-
-
-
-
-
-
-
-
-
Advertisement
-
Advertisement

 The kinetic energy necessary to accelerate an electron is:
The kinetic energy necessary to accelerate an electron is: For example, the Stanford linear accelerator can accelerate an electron to roughly 51 GeV [1]. This gives a gamma of 100,000, since the mass of an electron is 0.51 MeV/c² (the relativistic momentum of this electron is 100,000 times the classical momentum of an electron at the same speed). Solving the equation above for the speed of the electron (and using an approximation for large γ) gives:
For example, the Stanford linear accelerator can accelerate an electron to roughly 51 GeV [1]. This gives a gamma of 100,000, since the mass of an electron is 0.51 MeV/c² (the relativistic momentum of this electron is 100,000 times the classical momentum of an electron at the same speed). Solving the equation above for the speed of the electron (and using an approximation for large γ) gives: The de Broglie wavelength of a particle is λ=h/p where h is Planck's constant and p is momentum. At low (e.g photoelectron) energies this determines the size of atoms, and at high (e.g. electron microscope) energies this makes the Bragg angles for electron diffraction (co-discovered by J. J. Thomson's son G. P. Thomson) well under one degree. Since momentum is mass times proper-velocity w=γv, we have
The de Broglie wavelength of a particle is λ=h/p where h is Planck's constant and p is momentum. At low (e.g photoelectron) energies this determines the size of atoms, and at high (e.g. electron microscope) energies this makes the Bragg angles for electron diffraction (co-discovered by J. J. Thomson's son G. P. Thomson) well under one degree. Since momentum is mass times proper-velocity w=γv, we have For the 51 GeV electron above, proper-velocity is approximately γc, making the wavelength of those electrons small enough to explore structures well below the size of an atomic nucleus.
For the 51 GeV electron above, proper-velocity is approximately γc, making the wavelength of those electrons small enough to explore structures well below the size of an atomic nucleus.